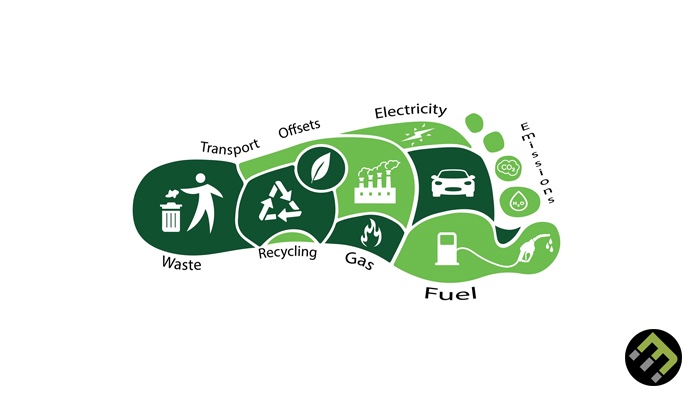
Sustainable Living: From Home to Lifestyle
In today’s world, where the effects of climate change and environmental degradation are more apparent than ever, the shift towards sustainable living has become not […]
Sustainable Living: From Home to Lifestyle